SOLUTION
WEB / Mobile
DCM (Demand Chain Management)
DCM is a demand network management system that performs orders/returns/collections/performance of wholesalers, salespeople, and consignment vendors.
-
Wholesale DCM
(Demand Chain Management)
Wholesaler Order/Return Management and Wholesale
-
Sales DCM
(Sales Management System)
Support salespeople, such as direct payment (hospitals, clinics/pharmaceuticals) orders/returns
-
Consigned DCM
(Partner Relationship Management)
Supports business activities of consignment vendors
Major features
-
Order/Refund
Order/return registration, order/return status check, collateral status check
-
Order management
Order management functions (item management, point of sale management, payment line management, Manage Shipping Plates)
-
Payment collect management
Collection processing (virtual account, card payment), collection statement inquiry, collection management
-
Client management
Ability to create, modify, and manage new accounts
-
Performance
Performance registration, performance statistics, and management features salespeople and consignors
-
Wholesale data
Wholesale sales, inventory data retrieval function
-
Sales status
Various sales data inquiry (customer, transaction statement, daily, Monthly sales status)
-
Sales Support
Support for handling travel expenses, daily expenses, and organizational expenses for salespeople
Features 1
SAP-based easy ordering system
It provides a simple web UI that allows users to easily order. It also offers a variety of SAP-based order management capabilities.
-
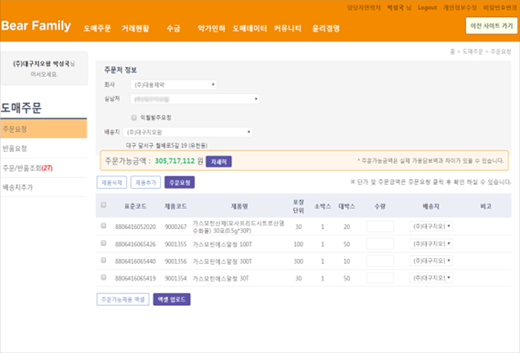
DCM Main Culture Plane for Wholesale
-
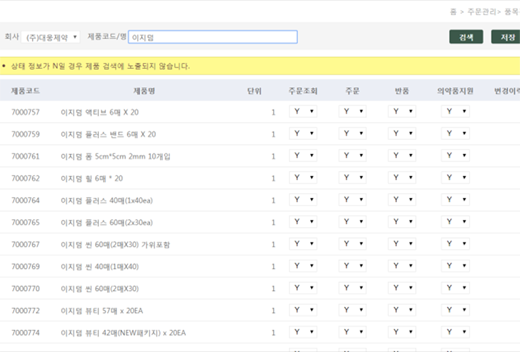
Sales DCM Item Management
Features 2
Provides UX-based convenience for Web & Mobile Support
We've made it possible to work anywhere, increasing efficiency. We also maximized convenience with a webUX base that is familiar to all users.
-
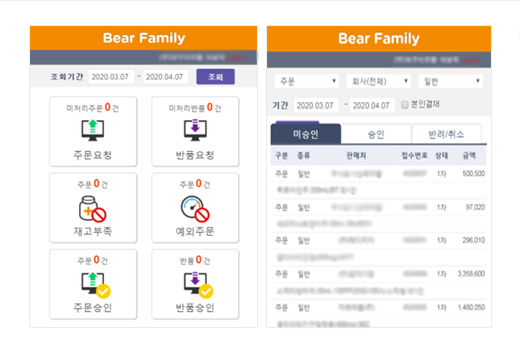
Wholesale DCM Mobile Main/Main Culture Area
-
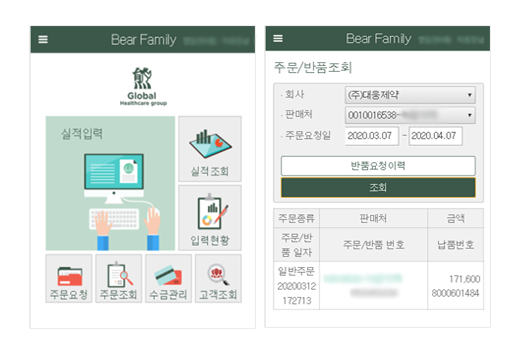
Salesperson DCM Mobile Main Screen
Features 3
SAP real-time revenue analysis
You can inquire the sales data of the ledger and statement in real time.
Helps you create an efficient marketing strategy with aggregated statistical capabilities.
-
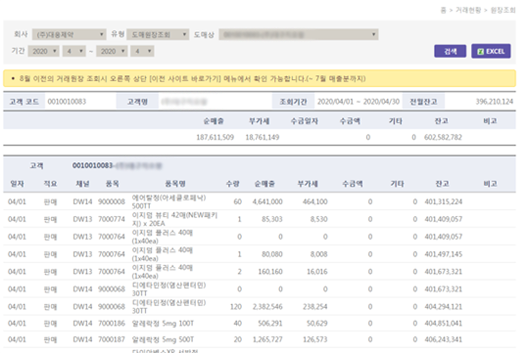
Consolidated DCM Transaction Statement
-
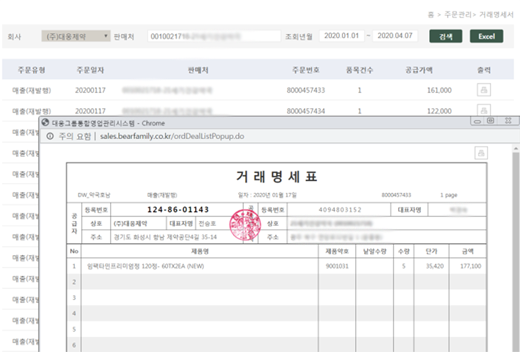
Salesperson DCM transaction statement
Features 4
Provide sales management system
Provides business support and management functions such as ordering, performance, and handling of unproven expenses for salespeople.
-
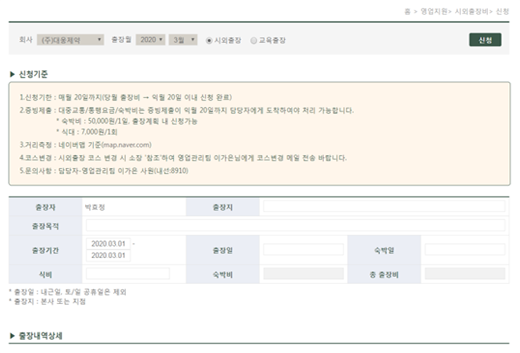
Salesperson DCM travel expenses
-
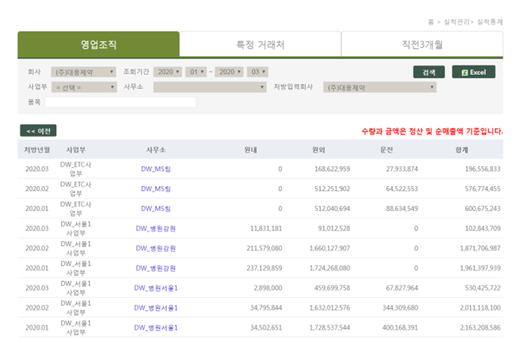
Salesperson DCM performance
-
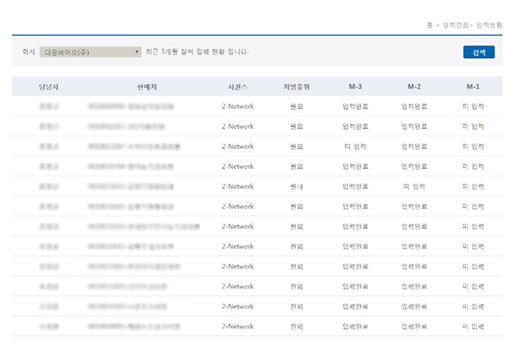
consigned DCM performance input status
-
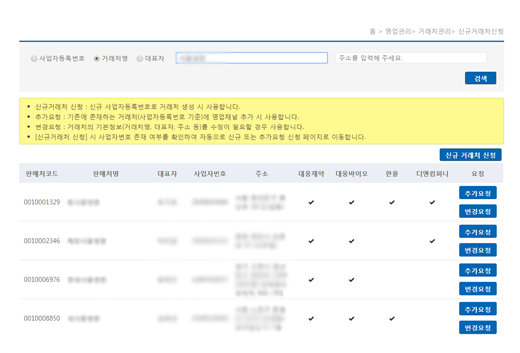
Subcontracting DCM Account Application
POP (Point Of Product)
Point of Product (POP) is a system necessary for sharing information and stabilizing quality and improving productivity through the control of process production progress and facility management by real-time management of information generated during manufacturing and production.
Major Features
-
Production order
Production/Virtual Order Management, SPLIT, Simultaneous Process Management
-
Production performance
Production/virtual order preparation/operating/cleaning and non-operating performance/manual real-time management
-
Supply management
Management of production orders and other orders, virtual orders, and absenteeism and tardiness management
-
Various numbers
Provide various indicators such as production progress, load analysis, lead time, yield, worker analysis, etc.
-
Outside system sync
SAP, BI, TMMS sync
-
Interface monitoring
Real-time external system interlocking results check and reprocess
Features 1
Provide real-time information by production process stage
Provides real-time on-site monitoring.
Facility non-operation analysis and response are possible.
-
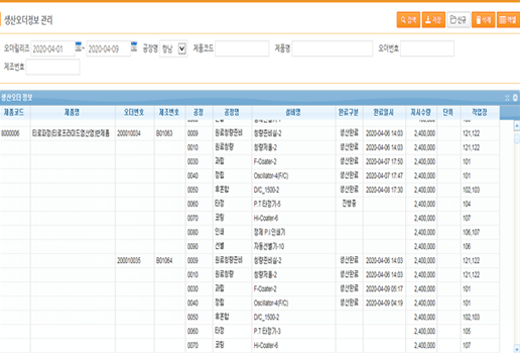
Production Order Information
-
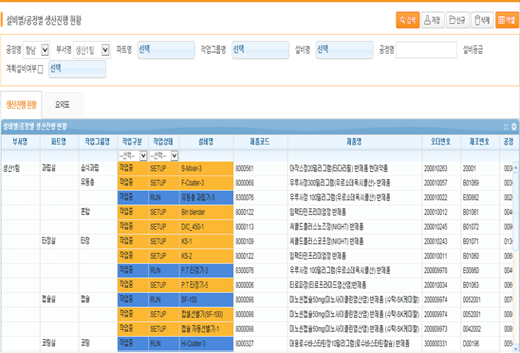
Production progress status by facility/process
Features 2
Support accurate production planning and purchase management by reflecting SAP real-time field information
Provides a web UI that allows easy monitoring real-time production order and input of production performance.
Also provides SAP I/F real-time monitoring and reprocessing.
-
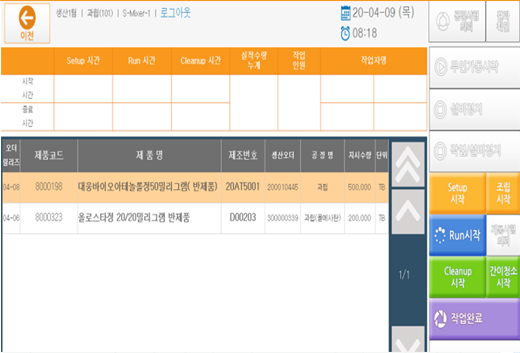
Site - Check Order and Register Performance (Real-time Reflection)
-
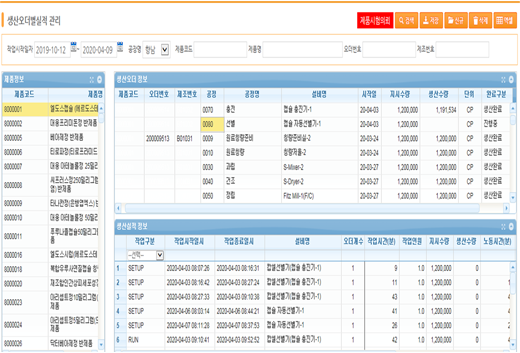
Performance management by production order
Features 3
Bidirectional interworking of LIMS through inspection request
Provides inspection, product inspection request, and decision results during the process.
Easy UI is provided for inspection requests.
-
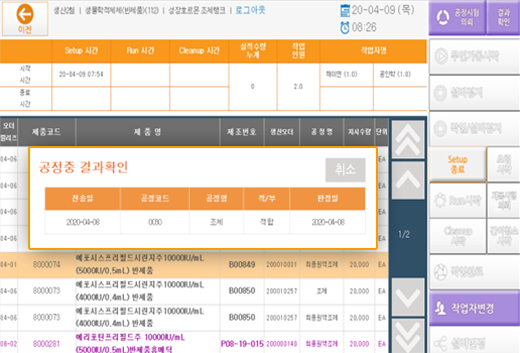
Requesting an inspection during the process/checking the results
-
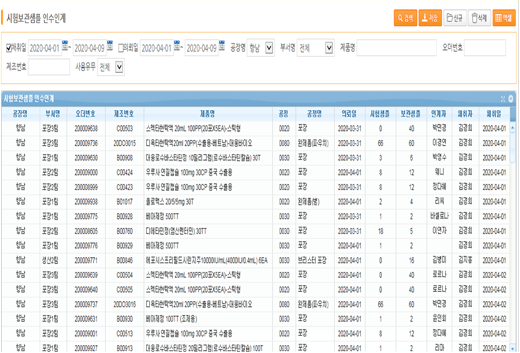
Current status of transfer of test storage sample
Features 4
Provide a variety of indicators to help identify trends and make decisions.
Provides more than 20 indicators including production progress, load analysis, lead time, yield, and worker analysis.
Also provides real-time, expanded report raw data with SAP BI interworking.
-
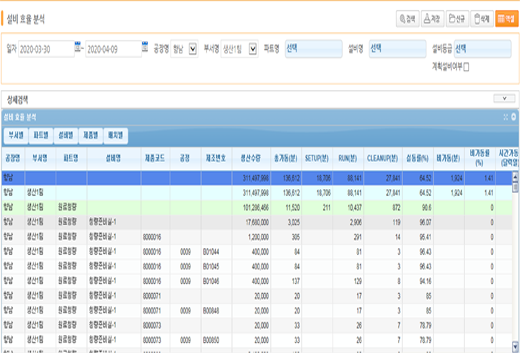
Facility Efficiency Analysis
-
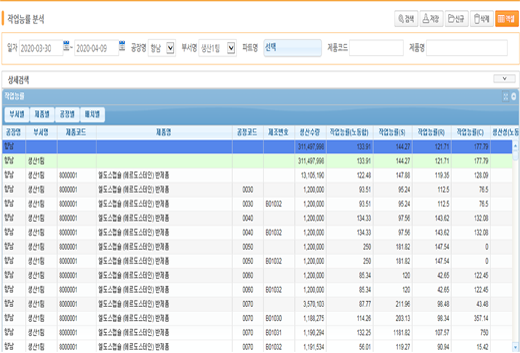
Operational Efficiency Analysis
Features 5
Functions for convenience and Performance Accuracy ↑
When entering performance, break time is managed so that work can be done conveniently without any additional shutdown.
In addition, enables convenient input of performance through simultaneous process management.
-
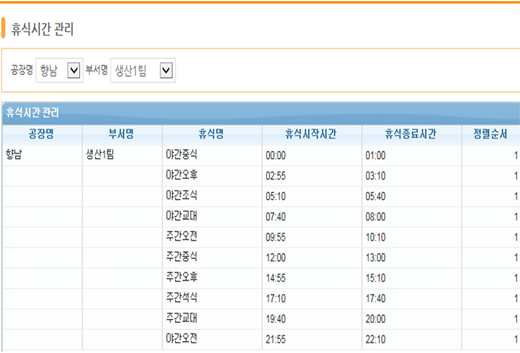
break time management
-
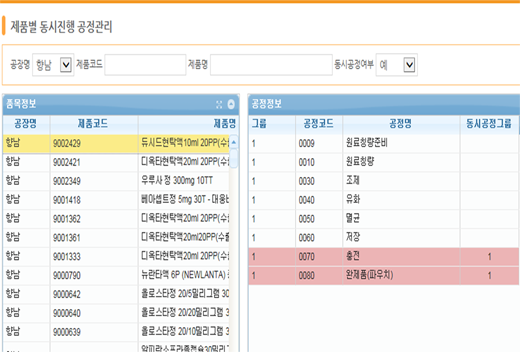
Simultaneous Process Management
LIMS(Laboratory Information Management System)
Computer system designed to manage various data generated in the laboratory and support Lifecycle of data including data collection, storage, analysis, reporting, and storage.
-
LIMS/ LES
( Laboratory Information Management System
Laboratory Execution System )Laboratory Information Management System
-
SDMS
(Scientific Data Management System)
Scientific Data Management System
-
LAS
(Laboratory Automation System)
Laboratory Automation System
Major features
-
Test
Processing test procedures (test reception, sample collection, test order, assignment of test subjects, input of results, review of results, final approval)
-
Test planning
Register and manage plans for stability testing and calibration/qualification assessment plans
-
Resources management
Manage the use and disposal of stored samples, stability samples, and the history of device use.
-
Reagent/Standard Product Management
Control the history of labelling, opening, disposing and using reagents and standard products used in testing
-
Settings management
Site-wide management with information processing on passwords, outputs, and Audit Trail
-
Master
Overall basic information management such as users, items, and specifications
-
SDMS
Feature to collect, classify, reuse, and manage data
-
LAS
Data Extraction Method Settings and Type-Specific Data Extraction Capabilities
Features 1
Test Process Management and Business Standardization
Systematized management of laboratory data input errors and laboratory business processes provides the ability to comply with U.S. FDA, GMP, and domestic and international legal regulations and ensure adequate quality.
-
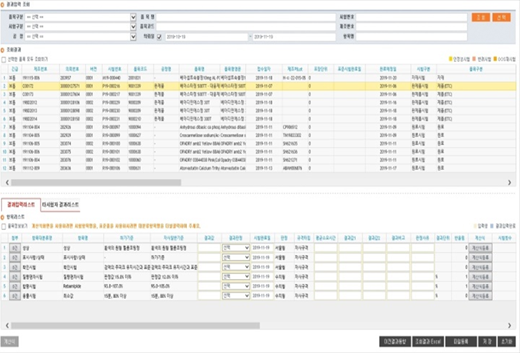
LIMS - Enter results
-
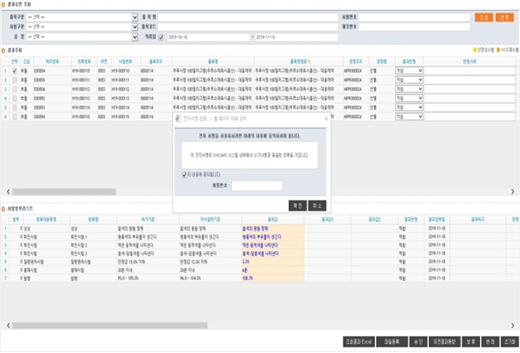
LIMS - electronic signature
Features 2
Automated test device data collection
It provides the ability to automatically process data transfer to other systems by collecting, storing, and processing data in conjunction with test devices.
-
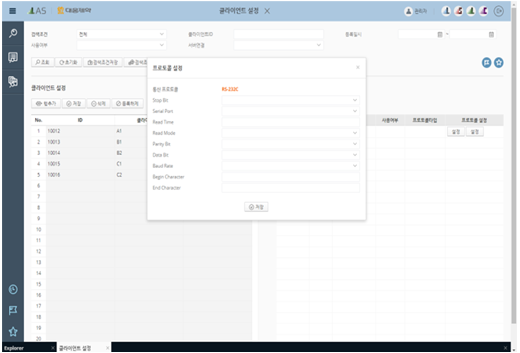
LAS-Client
-
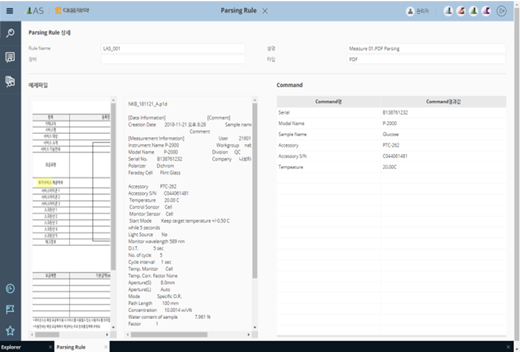
LAS-ParsingRule
Features 3
Laboratory Data Management
Documenting lab-generated raw data for safe storage, management, and utilization helps improve FDA's Guidance for Data Integrity compliance and work flow.
-
SDMS-Explorer
-
SDMS-Preview
